Prevedel lab
Developing advanced optical tools for modern life sciences
Fundamental insights in the biological sciences have often been stimulated and catalyzed by the development of new scientific tools, as these are the crucial technologies that drive new experiments and therefore lead to new discoveries. Light microscopy has revolutionized our understanding in many areas of biology, yet light scattering severely limits its performance and biomedical usefulness inside live, three-dimensional tissues, such as the mouse. New approaches and tools are thus required to noninvasively image biological function at depth inside living tissue with sufficient resolution, speed and contrast. The focus of our group at EMBL is to push the frontiers of deep tissue microscopy in terms of imaging depths and resolution by developing advanced and innovative optical imaging techniques. We also actively engage in developing and establishing unconventional imaging approaches such as Brillouin microscopy to ‘image’ mechanical properties of living tissues in a non-contact fashion and with diffraction-limited resolution in 3D.
To do so we draw from diverse fields such as multi-photon microscopy, active wave-front shaping, photo-acoustics, computational imaging as well as high-resolution spectroscopy.
The ultimate goal of our research is the direct application of our newly developed methods to fundamental and previously inaccessible biological questions, with an emphasis on the mouse model. Our multidisciplinary team comprises of physicists, engineers, computer scientists and biologists, and we engage in close collaboration with fellow groups within and outside of EMBL in the fields of cell and developmental biology as well as neuroscience.
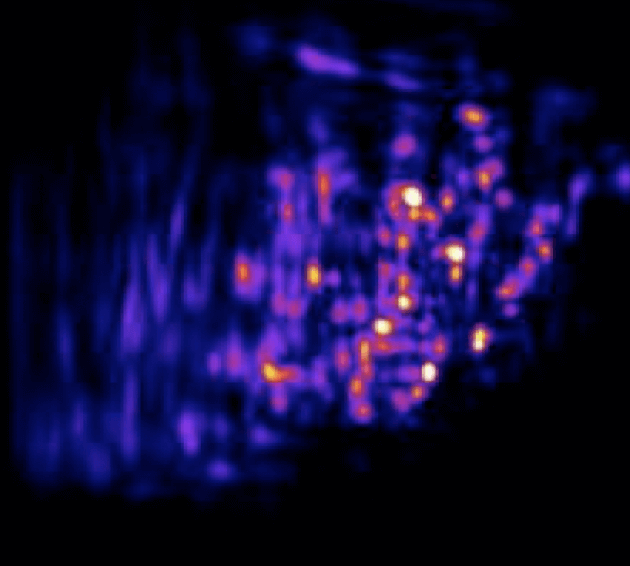
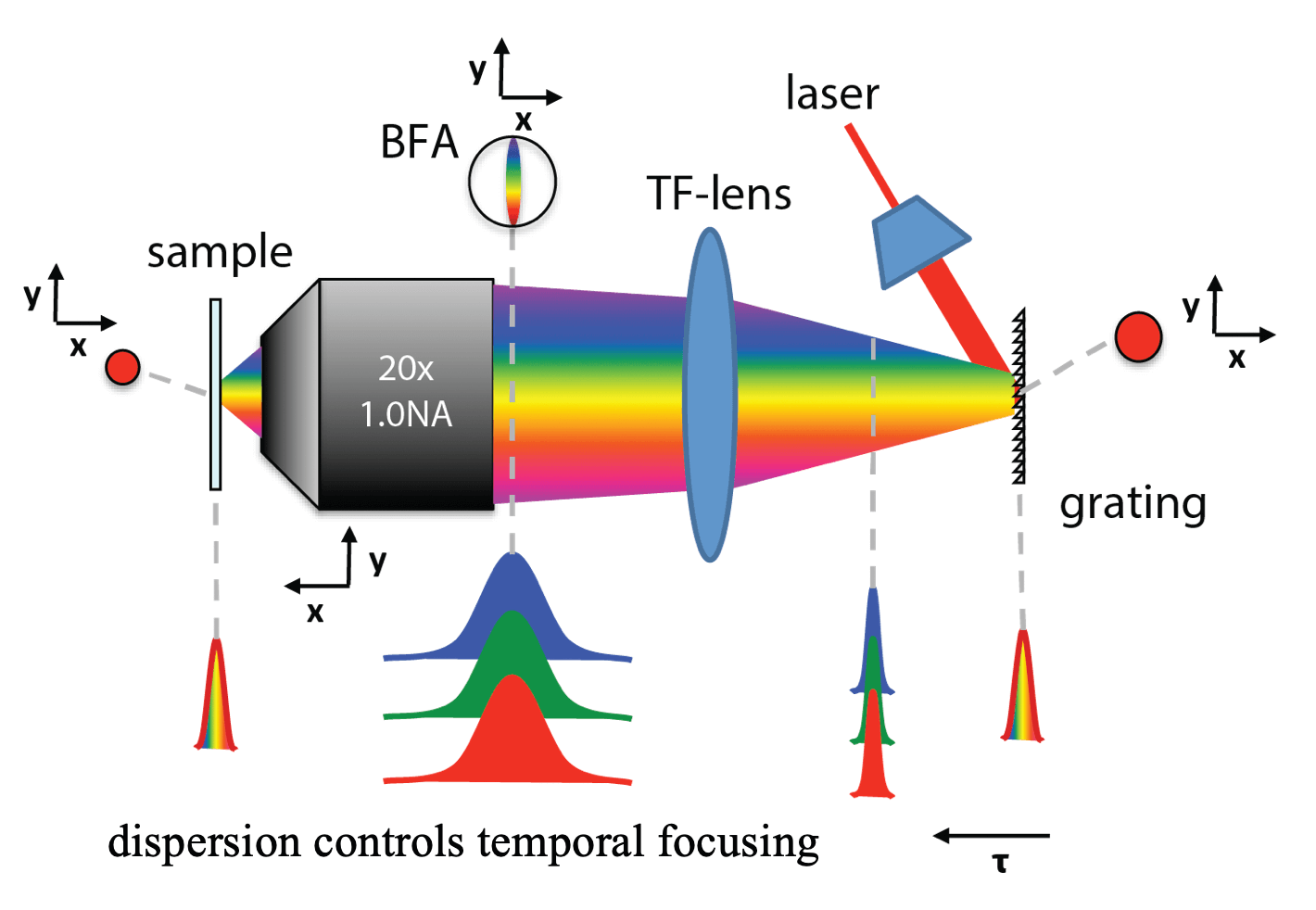
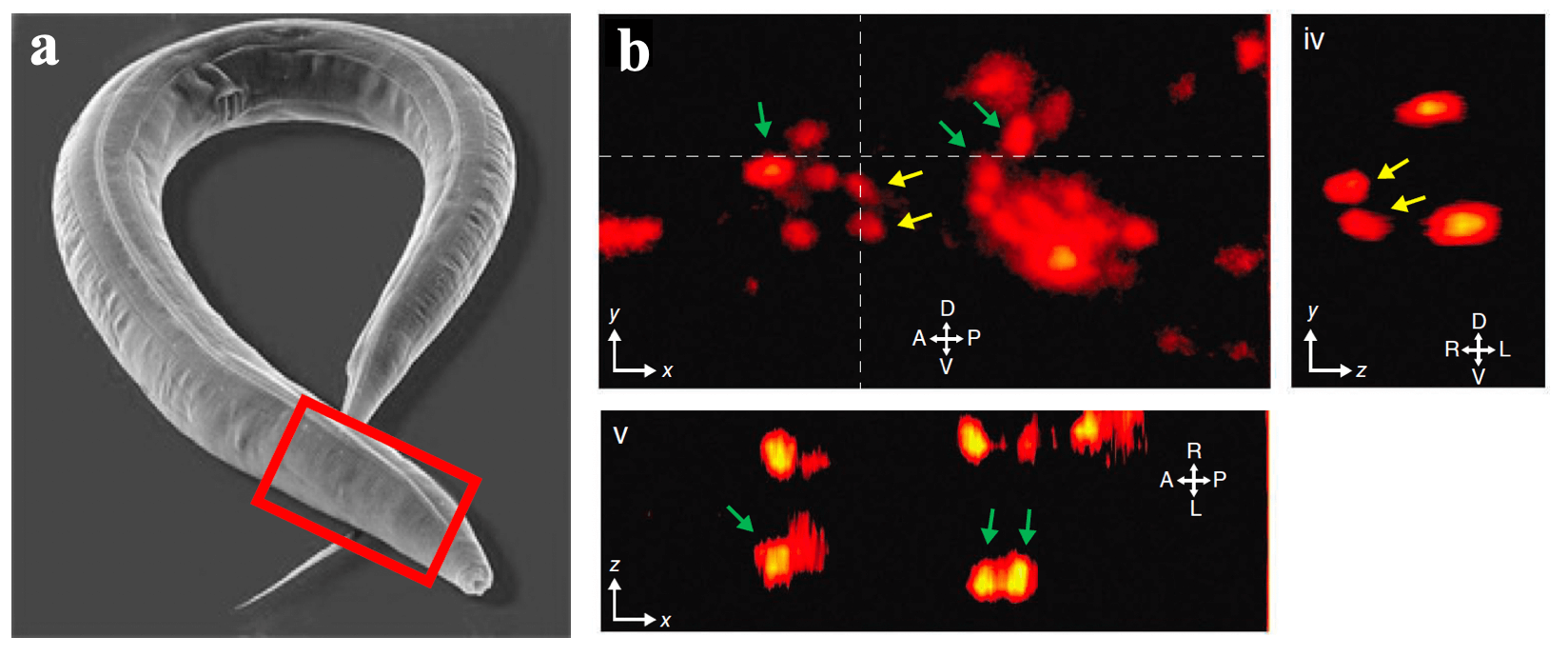
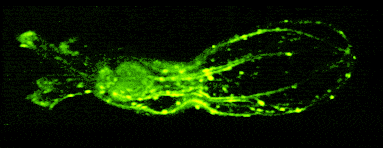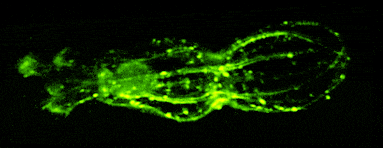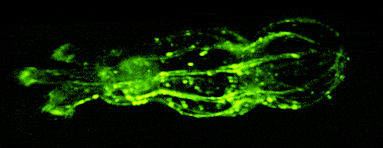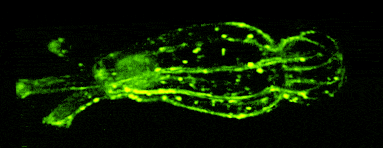
One of our past and current foci is the development of novel optical techniques for high-speed imaging. Together with our collaborators, we apply our methods to study neuronal activity and cellular dynamics in a range of model organisms. Amongst others, we have put forward a two-photon microscopy technique based on light-sculpting (Fig. 1) that has enabled the first whole-brain calcium imaging in C. elegans (Fig. 2). (Schrodel et al., 2013)
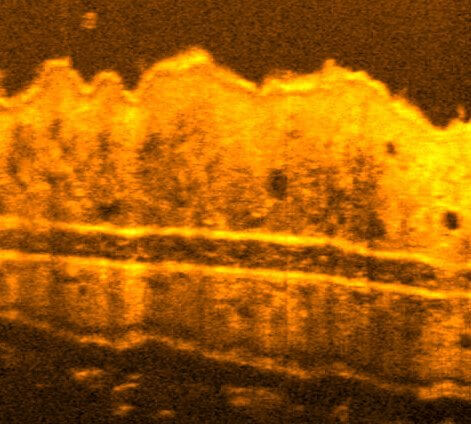
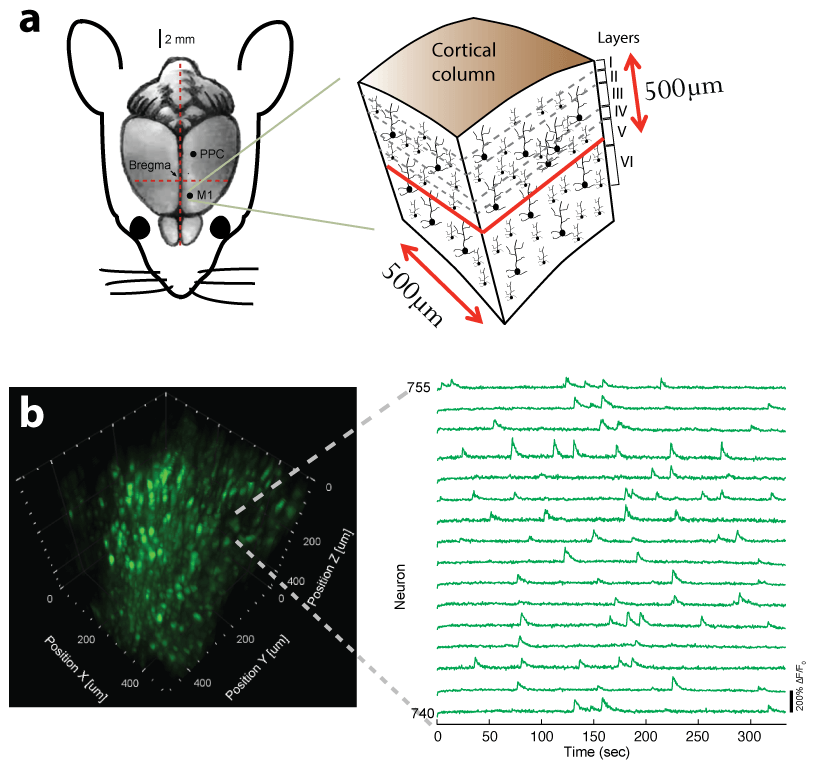
Recently, we extended our light-sculpting imaging methods to the scattering tissue domain and demonstrated fast volumetric calcium imaging across the majority of a cortical column in the mouse (Fig. 3). (Prevedel et al., 2016)
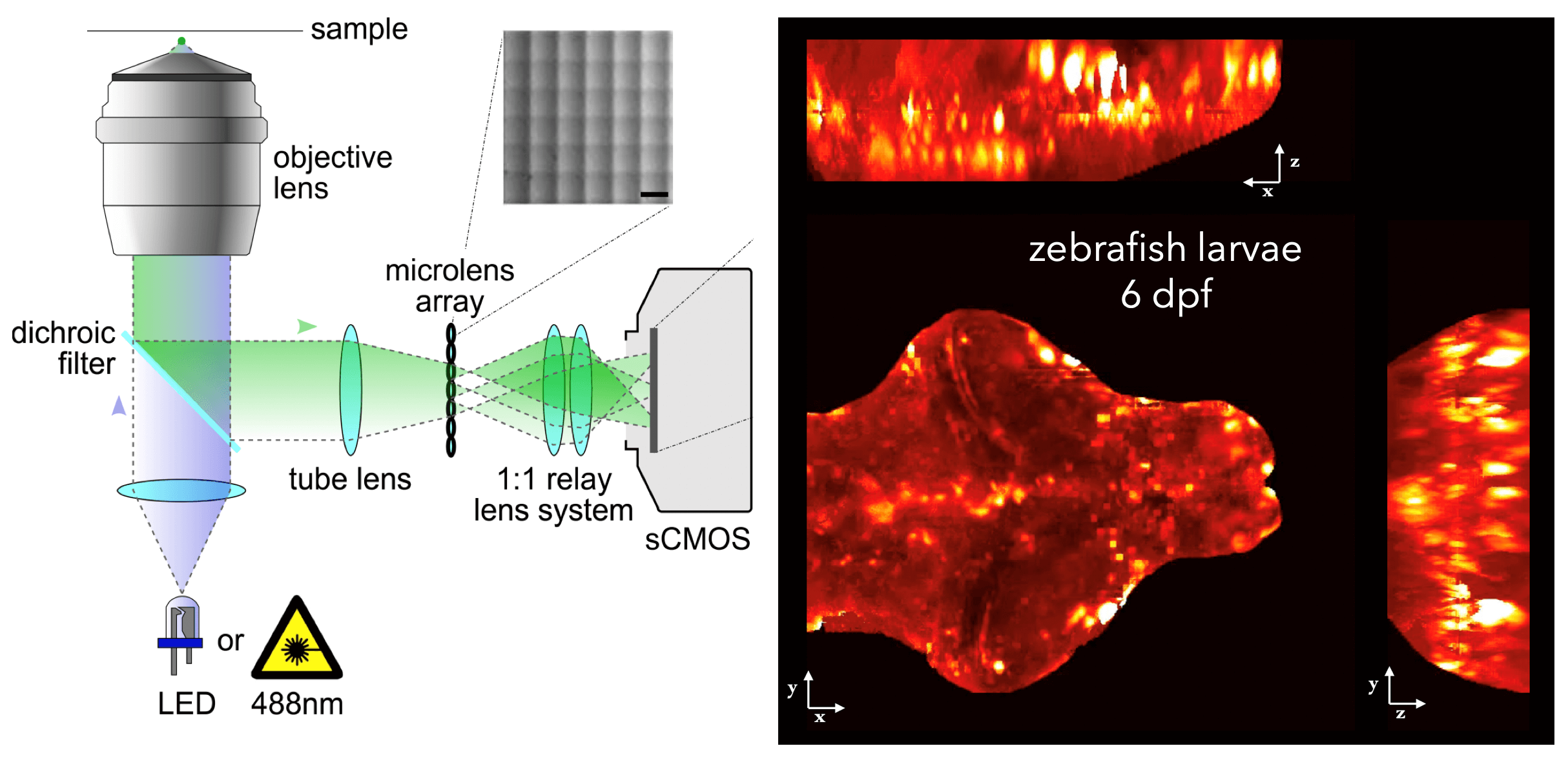
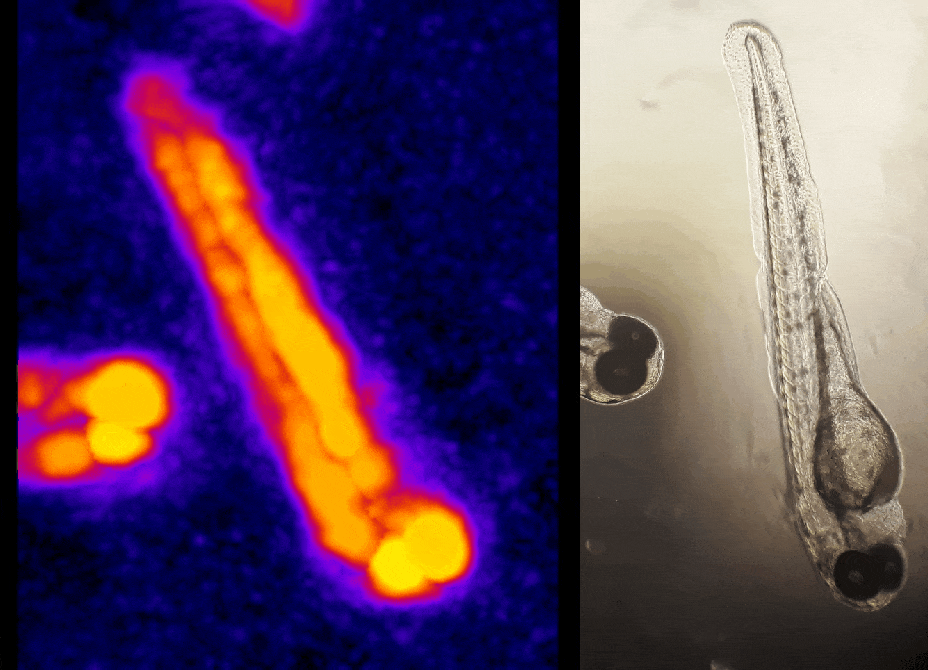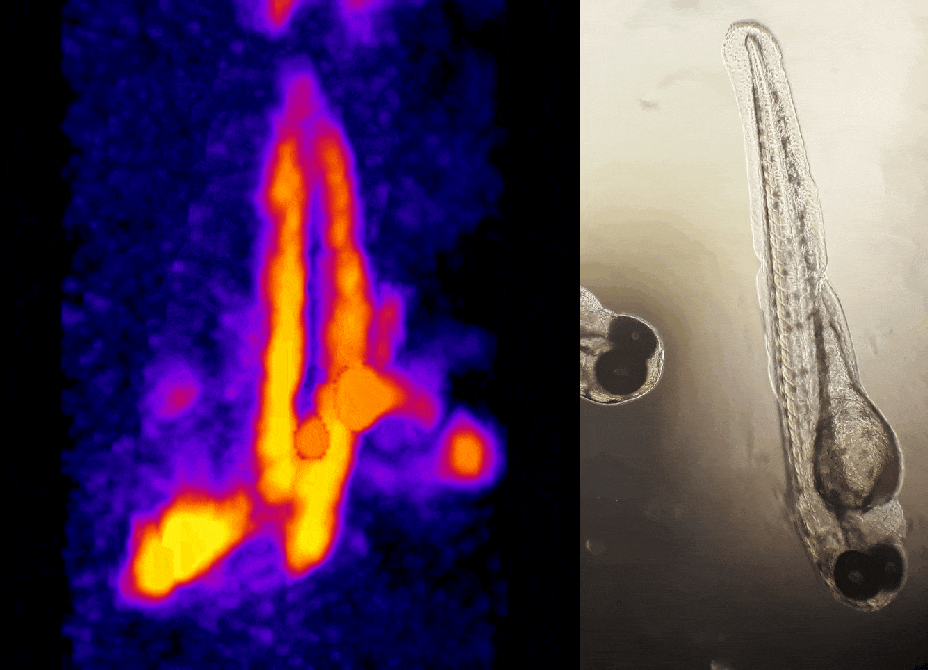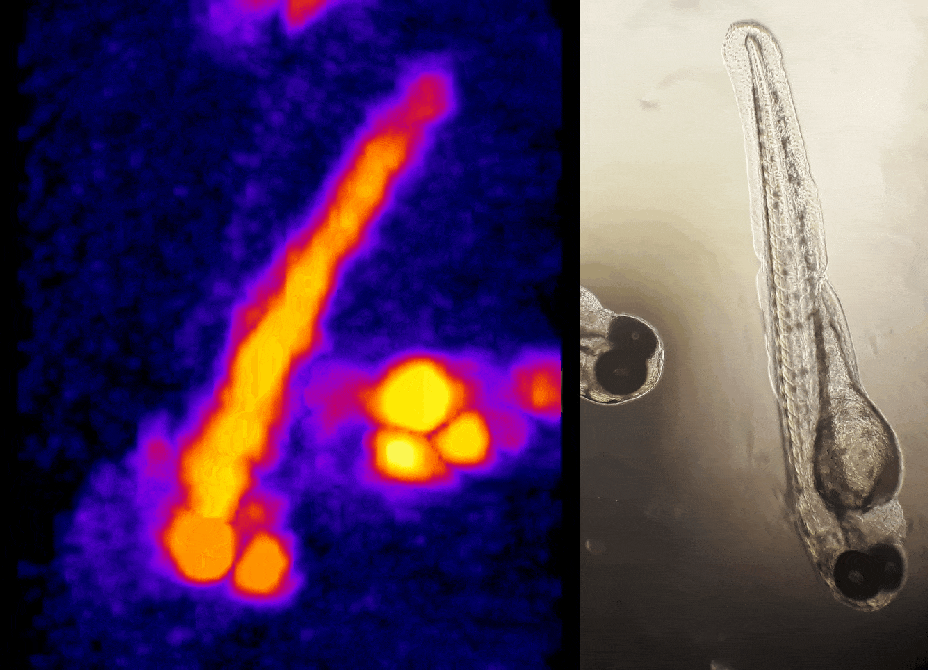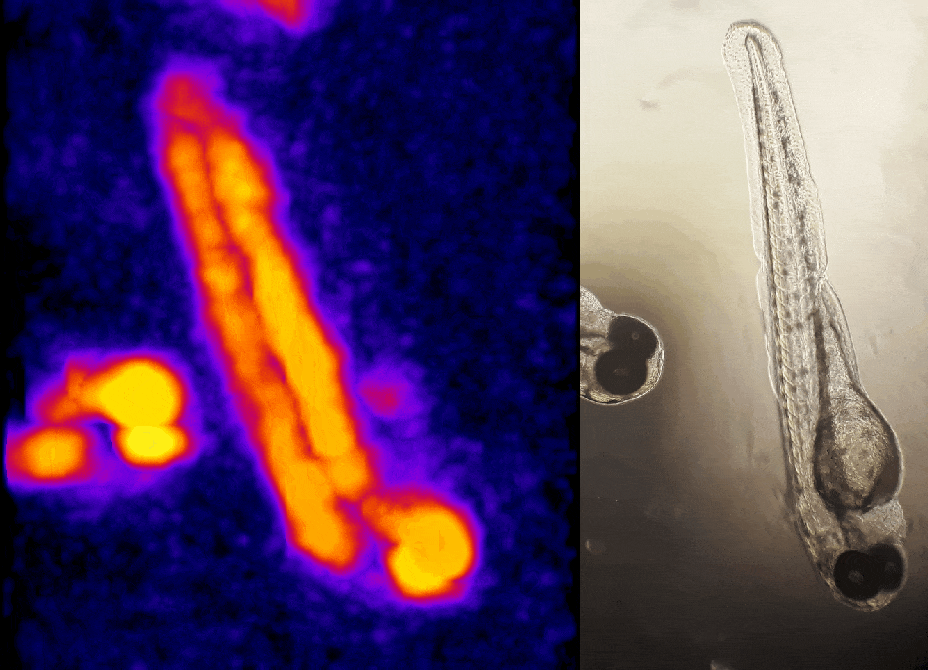
In other work, we have established light-field deconvolution microscopy, an elegant approach to perform volumetric imaging that achieves unprecedented acquisition speeds while requiring no mechanical scanning. This can be applied to image neuronal activity across entire, small organisms or small animal brains such as zebrafish larvae (Fig. 4). (Prevedel et al., 2014)
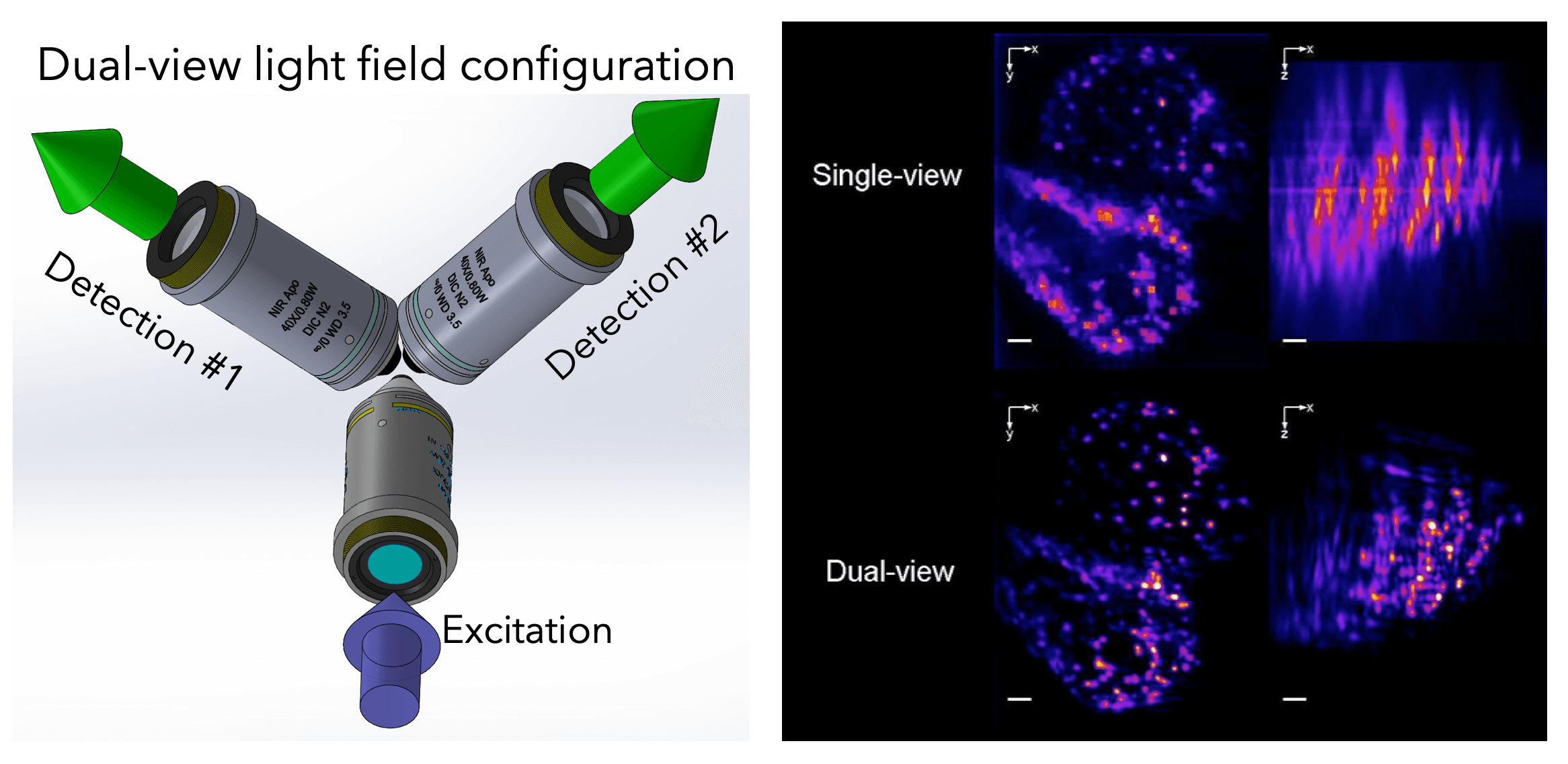
To further push the capabilities of light-field imaging, we have combined selective volume illumination with orthogonal light-field detection to obtain higher and more isotropic resolution, while significantly reducing reconstruction artefacts. With this we were able to image the beating heart of Medaka fish as well as its blood flow dynamics at up to 200Hz volume rate (Fig. 5). (Wagner et al., 2019)
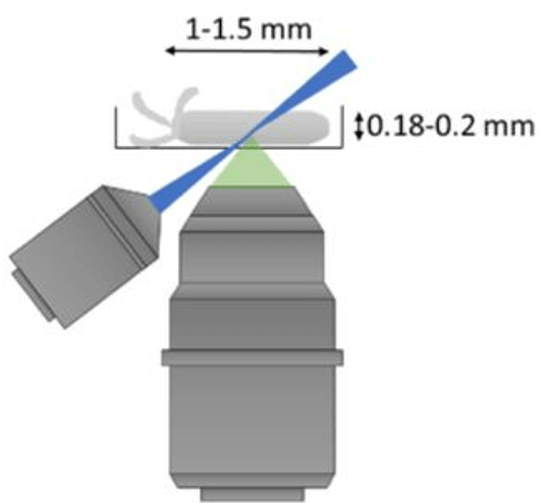
Recently, we developed an inverted light-sheet microscope based on oblique plane illumination for mesoscale imaging. The new system allows us to perform timelapse recordings of millimeter scale nematostella polyps with cellular resolution. (Singh et al., 2023)

Brillouin microscopy is a novel microscopy technique that measures mechanical properties such as elasticity and viscosity of biological samples in a 3D, nondestructive, and fully optical fashion.
Mechanical properties have been shown to play an important role in several biological processes, including control of malignancy in tumors, stem cells differentiation, as well as in morphogenesis of cells and tissues. Standard techniques in the field of mechanobiology, however, typically rely on external perturbations making them invasive, while other approaches often suffer from poor resolution. Instead Brillouin microscopy exploits a light-matter interaction, called Brillouin scattering, to probe mechanical properties in the GHz regime. Due to its all-optical nature, it can achieve, high, diffraction-limited resolution in 3D. (Bevilacqua et al., 2019)
The physical principle is outlined in Figure 1. Laser light is focused on the sample and interacts with sound waves, intrinsically present in any material because of thermal agitation. During the scattering process, the light experiences a positive or negative frequency shift. The amount of the shift is proportional to the speed of sound inside the material and is thus informative of visco-elastic properties. In particular, it is proportional to the longitudinal modulus, from which elastic and viscous parameters can be obtained. (Prevedel et al., 2019)
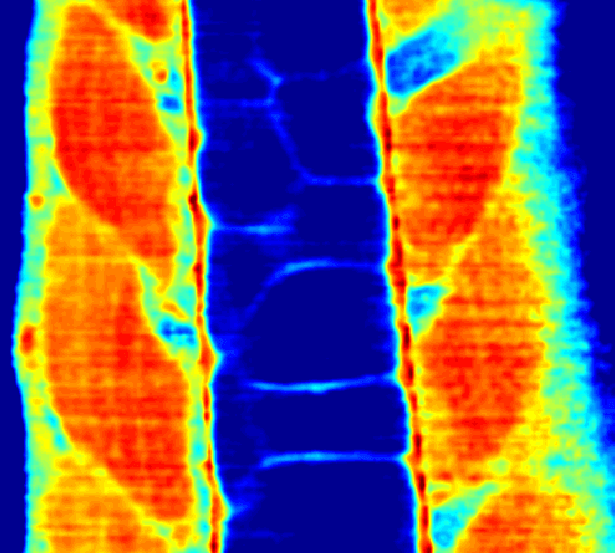
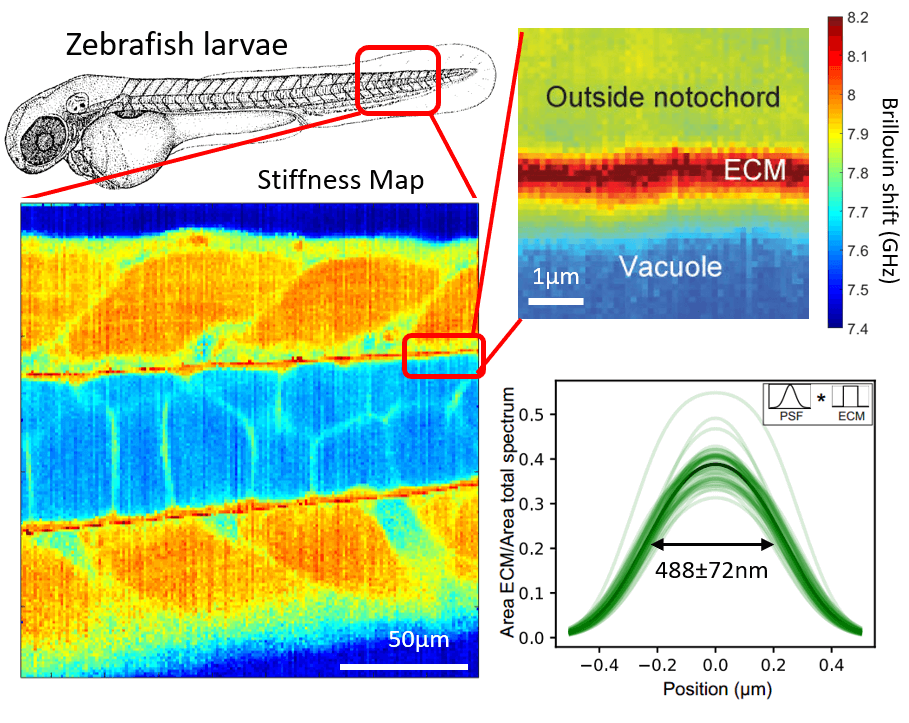
Our lab has developed a confocal Brillouin microscope capable of fluorescence co-detection for long-term imaging of biological processes. We are applying our Brillouin microscope, in collaboration with other groups, to interesting processes in development and cell biology. We optimized our Brillouin microscope to image the properties of sub-micron thick layers of extracellular layers in live zebrafish (Fig.2). (Bevilacqua et al., 2019)
In the past years we developed a line-scanning Brillouin microscope (LSBM) which improves the speed and reduced the photodamage compared to confocal implementations and allowed us to image developing living organisms in 3D. (Bevilacqua et al., 2023)
To further improve the effective speed and overall throughout of Brillouin imaging, we have developed a new method for highly multiplexed spectral acquisition using a custom-built Fourier-transform imaging spectrometer. This approach, termed FTBM, allows for full-field, two-dimensional Brillouin imaging with a throughput of up to 40,000 spectra per second, all while maintaining a high spectral precision. This represents a nearly three orders of magnitude improvement in speed and throughput compared to conventional confocal Brillouin microscopy (Bevilacqua and Prevedel, 2024)
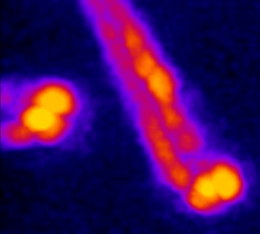
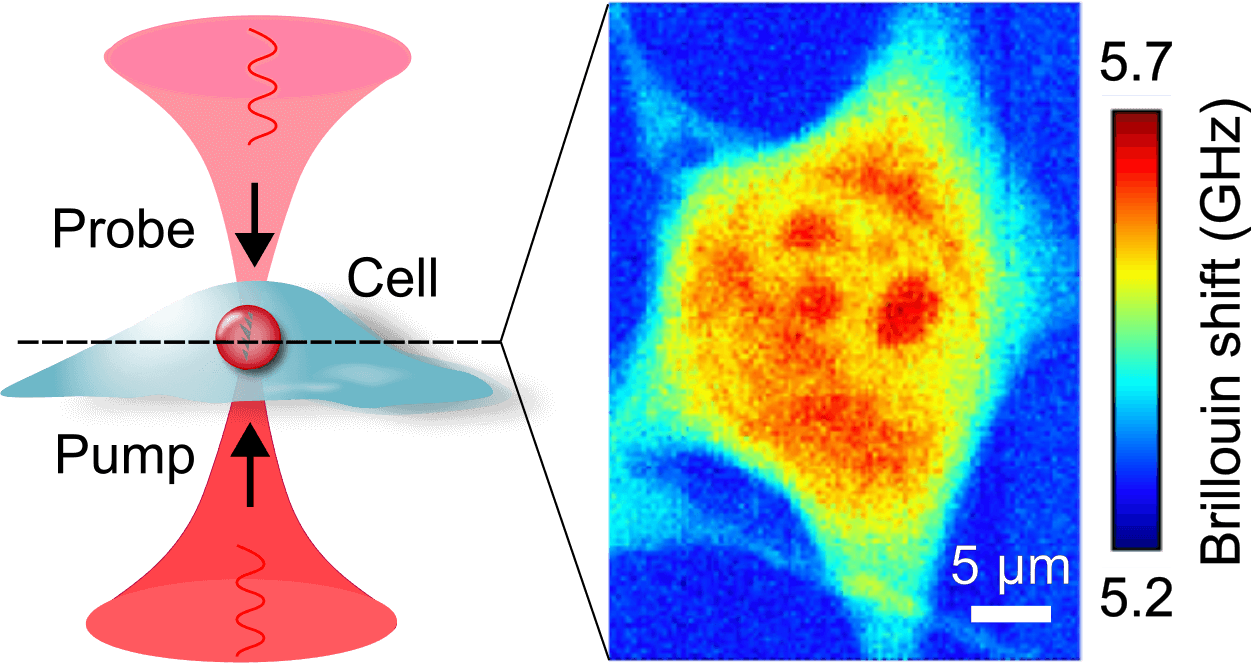
Recently we built a stimulated Brillouin microscope (SBM). In contrast to spontaneous Brillouin scattering, in SBS the acoustic waves are generated in the sample by two counterpropagating pump and probe beams (Fig. 3). We developed a pulsed version of the microscope (pulsed-SBS), which expoits the non-linearity of the signal intensity on the pump and probe optical power on the sample. We showed that we can reduce the power (or the exposure time) by a factor ˜20. (Yang et al., 2022)
In order to study dynamic biological processes in-vivo in mammalian organisms such as the mouse, techniques are required which enable non-invasive imaging at large tissue depth with sub-cellular resolution. Multi-photon microscopy is currently the technique of choice for in-vivo imaging of opaque and highly scattering tissue samples. However, scattering and optical aberrations lead to degradation of the point spread function (PSF) and hence, reduced image contrast, resolution and excitation power at depth. To enable imaging of cellular- and subcellular structures at high spatial resolution deep inside mammalian tissue in-vivo, we combine two powerful optical techniques: multiphoton microscopy and adaptive optics.

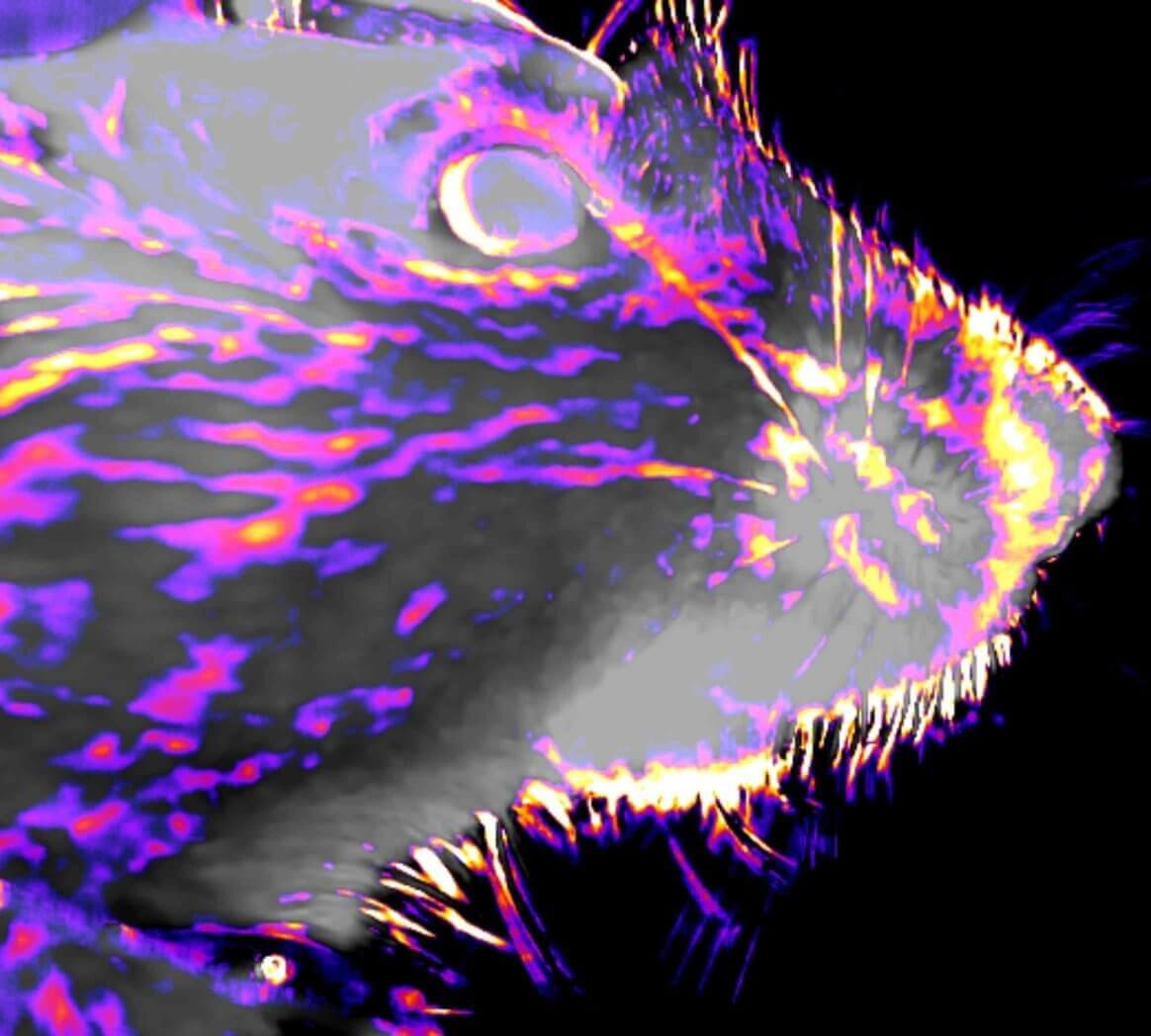
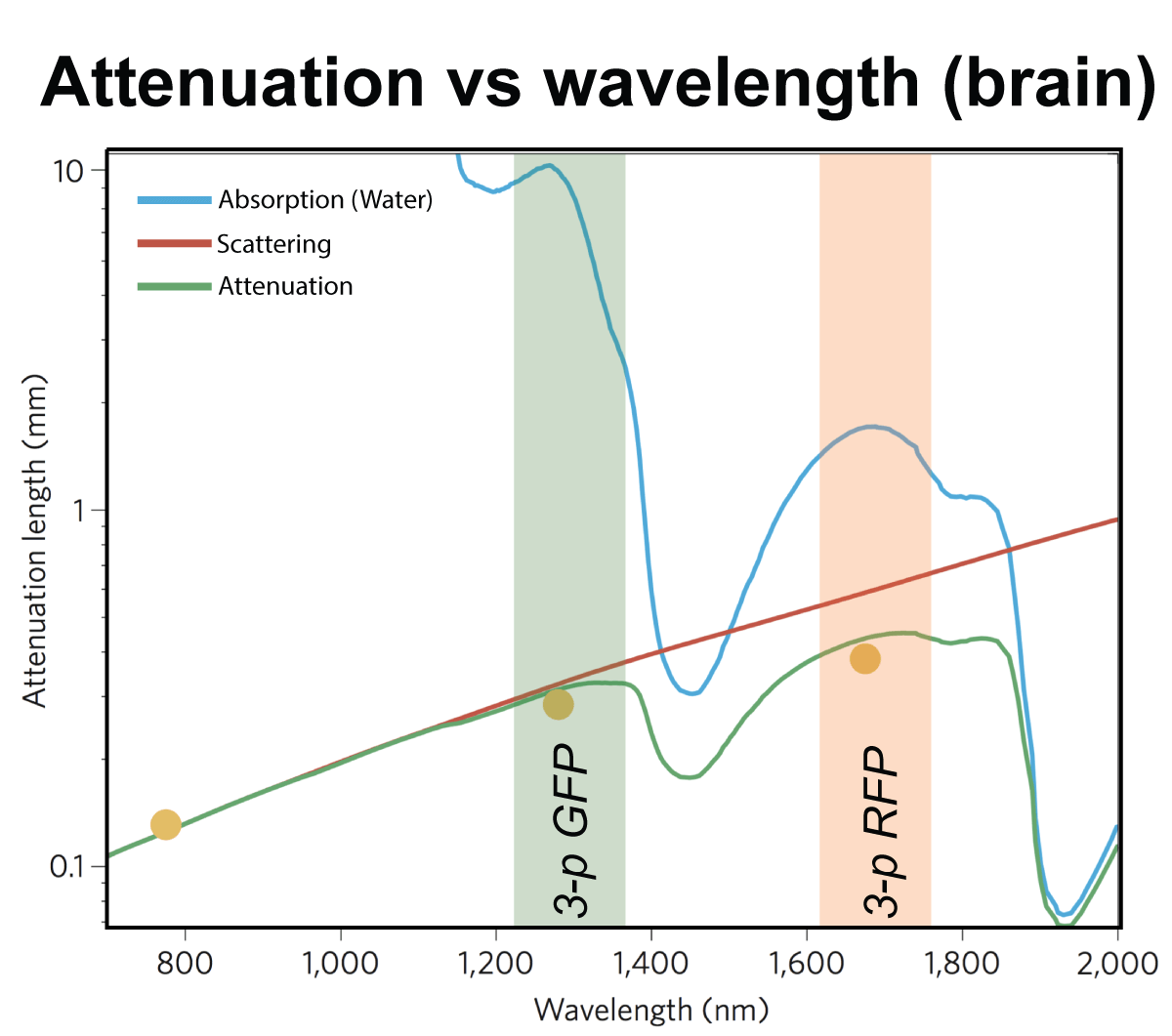
For deep tissue imaging we are exploiting the advantageous properties of three- photon excitation. The longer wavelength employed for excitation and the later on-set of out-of-focus fluorescence increases the signal-to-noise ratio at depth (Fig. 2). This can be combined with red-shifted fluorophores to further reduce scattering. (Qi et al., 2018)
Adaptive optics is a technique to improve imaging performance by correcting wavefront aberrations introduced by the biological sample and the optical system. We are working on direct and indirect wavefront sensing approaches to enable diffraction limited resolution deep inside the tissue (Fig. 3).
We apply our adaptive multi-photon technique to open questions at the forefront of biology and neuroscience.
The strong scattering properties of many biological tissues prevent deep imaging using visible light. Although approaches exist that partly circumvent this problem, such as multi-photon microscopy or Optical Coherence Tomography, they still hit a hard depth limit at about 1-2 mm in practice. To image beyond, we need to exploit other imaging modalities not based on classical light microscopy. One of the most promising techniques is photoacoustic imaging which allows to achieve imaging depth and resolution similar to that of ultrasound tomography while retaining molecular specificity.
In simple terms, the photoacoustic imaging is based on generating sound with the use of light (Fig. 1A). The use of high-energy, short laser pulses tuned to the absorption spectrum of the molecules of interest causes rapid transient heating of the tissue at the sites of light absorption. Rapid heating in turn causes local expansion of the tissue and generates propagating acoustic waves which can be used for ultrasound-based imaging. We are developing approaches based on photoacoustic tomography which allows to fully harvest the deep tissue imaging capabilities (<10mm) of the technique. In this approach, instead of acquiring images pixel by pixel, we excite the entire tissue at once and record the overall generated acoustic field over time from multiple directions. We then use a computational approach based on simulating the acoustic field propagation to reconstruct the absorbing molecule distribution from the acoustic field they generate (Fig. 1B).

Optical Coherence Tomography (OCT) is a label-free imaging technique that captures depth-resolved 3D views of biological samples by detecting back-scattered light, similar to ultrasound in principle. It is typically and more widely used for non-invasive structural imaging in medical research at mesoscale resolutions. Optical Coherence Microscopy (OCM) extends OCT into the microscopy regime by combining broadband illumination and spectrometers with high-numerical-aperture optics and, yielding micrometre-scale resolution while retaining OCT’s fast volumetric imaging and label-free contrast.
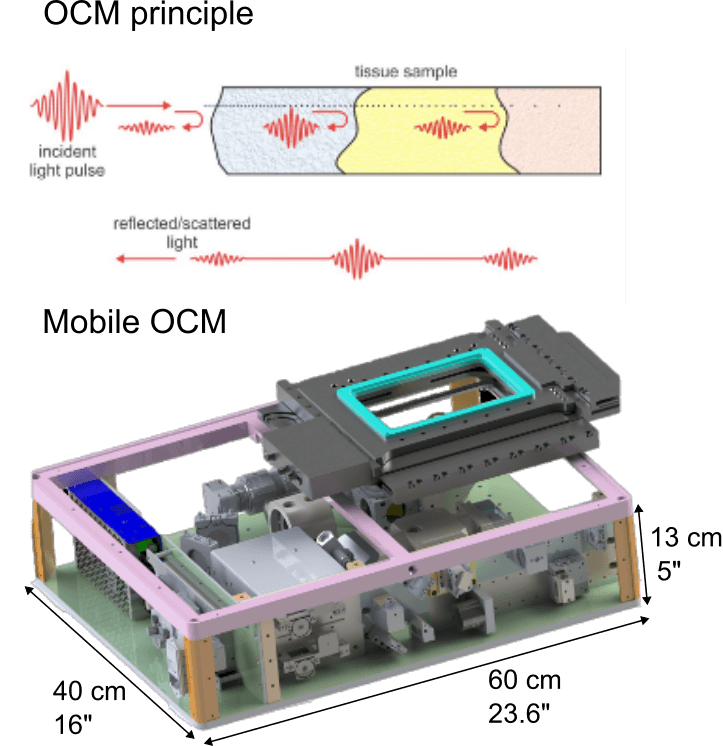
Recently, we have developed a dedicated, high-resolution OCM system for diverse life science applications. Specifically, we engineered a compact and mobile instrument that can be transported to remote, non-laboratory sites and field locations. Its portability allows high-resolution, 3D imaging directly in the field or near the point of collection, enabling immediate observation of delicate or short-lived specimens without the need for lab-based microscopes or any artificial fluorescence labelling. Our mobile OCM thus bridges laboratory-grade imaging and in situ applications, facilitating dynamic studies of living organisms in their native environments.
Specific tasks need specific tools. Our lab develops bespoke methods to unlock long standing questions in neurobiology. Currently our research is aimed at:
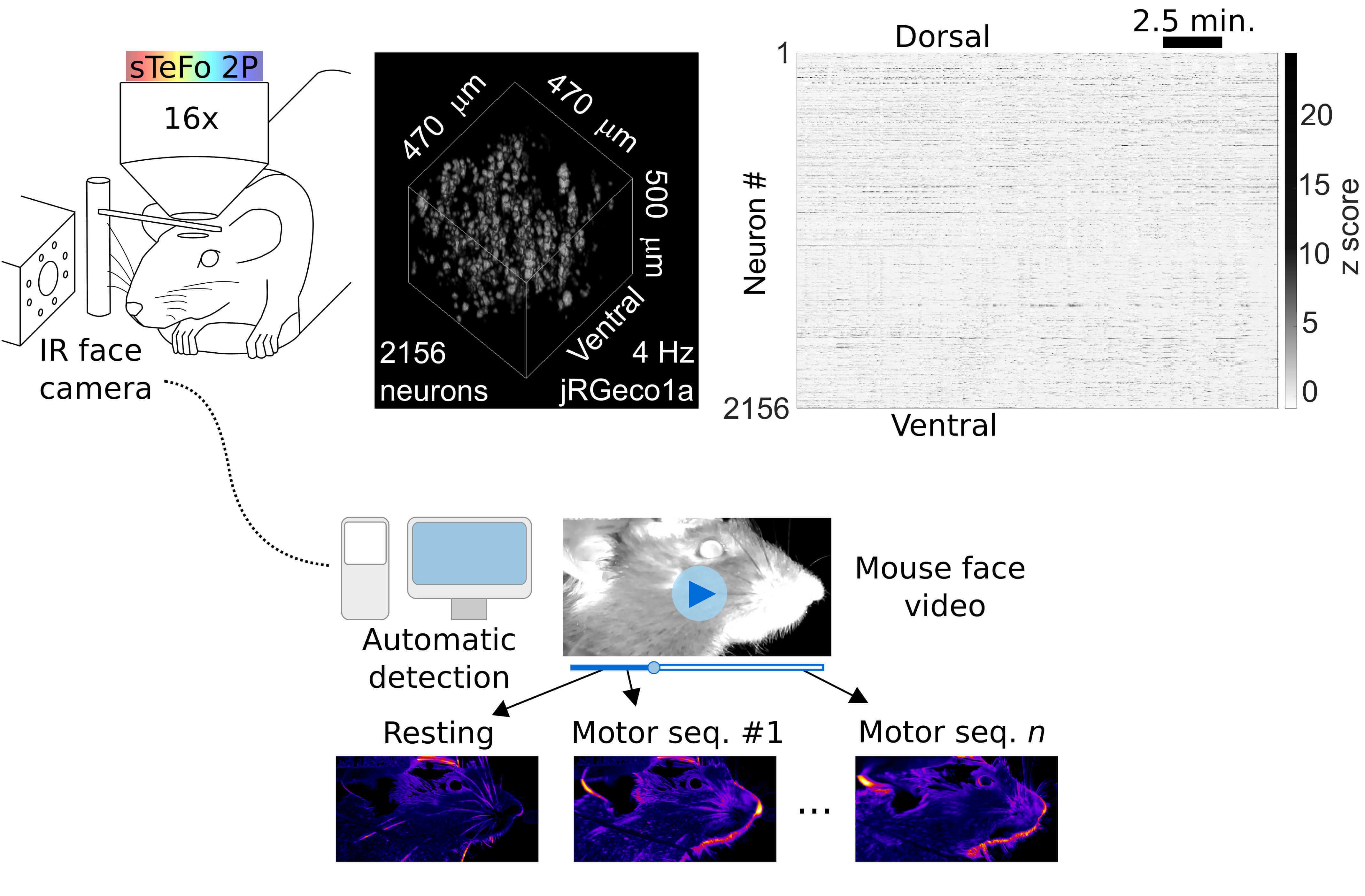
1) What is the neural basis of behavior? How does our brain control and generate such diversity of actions? Tackling this question requires tools that allow the interrogation of neural function at the systems level and, simultaneously, a comprehensive quantitative readout of complex behavior. To address this challenge, we have combined our high-speed bio-imaging system with unbiased videographic tracking of behavior (Fig. 1) which allows simultaneous sampling of behavioral sequences and neuronal population activity in head fixed but awake and behaving mice. We developed an unbiased, unsupervised image analysis algorithm to quantify on-going facial motor behavior in head fixed mice (GitHub), which enabled us to find a functional link between neuronal population activity at the motor cortex and face movements. (Boffi et al., 2021)
2) One of the key features of our brains is its ability to categorize sensory inputs. For example, we easily attribute different meaning to the sounds of the words ‘pea’ and ‘tea’, or to sounds coming from the left or the right side of the head versus the front when we are walking across a street. However, if we are passively listening to a lecture about food and drinks, we might find periods where we can’t figure out if the speaker is talking about peas or tea. Equally so, the lecturer might have a hard time locating the sound of a student phrasing a question from the left or right side of the auditorium. To interrogate the mechanisms by which neuronal populations reliably categorize auditory inputs we modified our fast volumetric sTeFo 2P Ca2+ imaging platform to include a fully automated ultrasonic speaker system which delivers auditory stimuli from flexible directions to ultrasonic hearing animal models like mice.
Building a microscope requires adequate control software to synchronise all the different parts. This is typically written in-house or adapted from open-source community efforts.
To aid in the democratisation of these tools and enable higher flexibility, we are working together with the EMBL-based company Suricube, founded by Lars Hufnagel, to develop an FPGA-based platform that features GHz sampling and processing capability for advanced light microscopy applications.
The communication with the workstation will be done though MQTT, an IoT-protocol, allowing the development of custom open-source GUIs tailored to a specific microscopy implementation and integrated in the existing software ecosystem.

Carlo Bevilacqua
Optical Engineer
Working on...

Samuel Davis
ARISE PostDoc
Working on...
Júlia Ferrer Ortas
ARISE PostDoc
Working on...

Juan Manuel Gomez
PostDoc
Working on...
Frederik Görlitz
PostDoc
Working on...

Sebastian Hambura
Software Developer
Working on...

Markus Körbel
PostDoc
Working on...

Jinhao Li
PhD student
Working on...

Octave Martin
PhD student
Working on...

Tabea Quilitz
PhD student
Working on...

Nicolae Turcan
PhD student
Working on...
Laura Campama
Researcher shared with Kreshuk and Crocker groups
Working on...

Chen Liang
ARISE with Zimmermann Team
Working on...

Azqa Ajmal Khan
PostDoc shared with Jechlinger group @ MOLIT
Working on...

Hyoungjun Park
Predoc shared with Kreshuk group
Working on...

Christopher Randolph Rhodes
ARISE with Pepperkok Team
Working on...
| Name | Period/position in the lab | Position after EMBL |
|---|---|---|
| Juan Boffi | 03/19 - 12/25 PostDoc |
Imaging Specialist, Crick Institute, UK |
| Ling Wang | 09/16-12/25 Research scientist |
Professor, Jiliang University, China |
| Nikita Kaydanov | 10/20-08/25 PhD student |
|
| Gretel Kamm | 03/22-06/25 PostDoc |
Lab manager, University of Alicante, Spain |
| Lucien Rauscher | 10/23-09/24 Master student |
Predoc, Abbelight , France |
| Ivo Leite | 05/21-06/24 PostDoc |
Engineer, Bosch, Germany |
| Marina Sabbadini | 01/21-03/24 PhD student |
|
| Amr Tamimi | 02/21-10/23 PostDoc |
Optical Engineer, LaVision, Germany |
| Kaushikaram Subramanian | 03/20-06/23 EIPOD |
Optical Engineer, Zeiss, Germany |
| Emanuel Maus | 01/21-03/23 Master student |
Software engineer, Fraunhofer-Institute, Germany |
| Fan Yang | 09/20-12/22 PostDoc |
Group leader, Shanghai Institute of Optics and Fine Mechanics, China |
| Jing Zhou | 01/22-09/22 Visiting Predoc |
Predoc, Zhejiang University, China |
| Jakub Czuchnowski | 09/16-05/22 PhD student |
PostDoc, Harvard University, USA |
| Rajwinder Singh | 09/17-03/22 PhD student |
Researcher, LaVision, Germany |
| Ivana Salerno | 01/21-07/21 Master student |
Predoc, Goustave Roussy hospital, Paris |
| Lina Streich | 09/16-06/21 PhD student |
Researcher, Luxendo, Germany |
| Senthilkumar Deivasigamani | 01/17-02/21 EIPOD |
Research Associate, National Heart and Lung Institute, UK |
| Chii Jou Chan | 06/20-12/20 PostDoc |
Group leader, Mechanobiology Institute, Singapore |
| Ronja Rehm | 05/19-10/20 Master student |
Predoc, University of Göttingen, Germany |
| Tristan Wiessalla | 10/19-08/20 Master student |
Predoc (Asari lab), EMBL Rome, Italy |
| Mehmet S. Ozturk | 01/17-12/19 PostDoc |
Assistant Professor, Karadeniz Technical University, Turkey |
| Matteo Barbieri | 07/19-12/19 Visiting student |
Visiting student, Harvard University, USA |
| Nils Wagner | 04/17-09/19 Master student |
Predoc, TU Munich, Germany |
| Laura Steffens | 05/19-08/19 Intern |
Visiting student, Chicago University, USA |
| Momoko Kawarabata | 02/19-07/19 Master student |
Lab Assistant, Hampton University, USA |
| Claudia Robens | 06/18-08/18 Intern |
Student, Heidelberg University, Germany |
| Dongyu Li | 05/18-07/18 Visiting Predoc |
Postdoc, Huazhong University, China |
| Kris Krupp | 10/17-04/18 Intern |
|
| Dmitry Richter | 09/16-12/17 Master student |
Predoc, Harvard University, USA |
For more information please visit this site.
We are running a Brillouin microscopy course
05/02/2026We're exciting to run the EMBL Course on Brillouin microscopy next week, the second time after 2018. The course is aimed at researchers at all levels who are applying or planning to apply Brillouin microscopy to their research and wish to acquire more knowledge and hands-on training on the technology.
Funding news
27/01/2026Exciting news - we’ve received an ERC Proof of Concept grant! This will help translate our ERC funded research on developing advanced Brillouin imaging tools into real-world applications.
Ling and Juan are leaving the lab
11/12/2025Farewell to our long-time lab members Ling Wang and Juan Boffi, who are taking up exciting new positions in China and the UK, respectively. All the best for your new career chapters and stay in touch!
New lab members
01/10/2025Today we welcome two newcomers to the Prevedel lab: Nicolae Turcan is starting a PhD together with the Kreshuk lab on smart microscopy, while Georg Tauts will work on photoacoustic projects for his MSc thesis. Welcome Nicolae and Georg!
Frederik Görlitz joining our lab as PostDoc
15/05/2025Frederik will be working on our CZI project that combines light and sound for deep imaging. Welcome Frederik!
Robert was interviewed by Wiley Analytical Science
31/03/2025Wiley Analytical Science published an interview with Robert, descibing what the lab is currently working on.
Nikita defended his PhD thesis!
26/03/2025Congratulations to Nikita for an outstanding PhD defense!
Paper news!
20/02/2025We're excited about our recent publication on Full-field Brillouin microscopy! You can also read about it in the EMBL news.
Our Optical Coherence Microscopy is travelling alongside the TREC expedition
17/12/2024Our Optical Coherence Microscopy was optimised to fit in a suitcase and partecipated to the TREC expedition. You can read more about it in the EMBL news.
Markus Körbel joining our lab as EIPOD
01/10/2024Markus will be working on imaging brain with Brillouin microscopy. Welcome Markus!
We are at "BioBrillouin 2024" in Trondheim!
06/09/2024We're excited to present at the annual BioBrillouin in Trondheim next week. Check out talks from Carlo Bevilacqua, Juan Gomez and a poster from Jinhao Li!
Official websiteNew lab members!
01/09/2024Tabea Quilitz joins us from a MSc with Jan Huisken's lab to pursue a PhD in our lab. Azqa Khan (shared postdoc with Jechlinger group @ MOLIT) joins us to work on our joint project to further develop 4D organoid imaging methods. Welcome Tabea and Azqa!
People from MPI Singapore are visiting our lab
28/08/2024We welcome Kim and Jake who are visiting us from MBI Singapore and our collaborator Chii Chan's group. They will spend some weeks with us to use our OCM and Brillouin technologies.
Paper news!
26/08/2024We're excited about our recent publication reporting novel photoacoustics probes optimized for calcium imaging in mouse brains. Wonderful collaboratin with Claire Deo's group at EMBL. Congrats Alex, Nikita, Juan and Gretel and everyone else!
Funding news
01/05/2024We're excited to receive funding from the Chan-Zuckerberg Initiative to continue our research on advanced deep tissue imaging technologies via a Phase 2 grant! This is an interdisciplinary collaboration with the Deo lab at EMBL, the Gigan and DeAguiar lans in Paris as well as the Stiel lab in Munich. Currently looking for postdocs!! You can apply here, if you are interested.
Our Optical Coherence Microscopy contributed to understanding sponge movement
25/01/2024Our Optical Coherence Microscopy contributed to revealing an ancient relaxant-inflammatory response mechanism that gets sponges moving. You can read more about it in the EMBL news.
We are at "BioBrillouin 2023" in Dublin!
06/12/2023We're excited to present at the annual BioBrillouin in Dublin this week. Check out talks from Juan Gomez, Fan Yang, and a poster from Carlo Bevilacqua!
Official websiteOur work on Brillouin microscopy has been featured by the BioPhotonics magazine
15/11/2023You can read more abut it in the November/December 2023 issue. Check it out here.
Our work on pulsed stimulated Brillouin scattering got published online!
26/10/2023We're proud and happy about our latest work in Brillouin Microscopy in which we were able to markedly reduce laser powers in stimulated Brillouin scattering, thereby enabling new applications to sensitive biological samples. Congratulations to everyone involved!
We are presenting at "Seeing is believing" conference!
04/10/2023Check out Carlo's talk on Brillouin Microscopy (Friday 6th, 11:25), Amr's flash talk and poster on Multiphoton Microscopy (#210), Ling's poster on Optical Coherence Microscopy (#227), Nikita's poster on Photoacoustic Tomography (#125), Júlia's poster on Multiphoton Microscopy (#87).
Official websiteFunding news
01/10/2023We're thrilled to receive a Stimulator grant from the Heidelberg-Mannheim Life Science Alliance to work on bridging temporal and spatial scales in mouse brain imaging together with the Venkataramani lab from Heidelberg University.
Jinhao Li joining our lab as PhD student
01/09/2023Jinhao will be working on stimulated Brillouin microscopy. Welcome Jinhao!
MIT Technology Review article on our lab's work
31/05/2023MIT Technology Review published an article on our lab's work on Adaptive Optics, Photoacoustics and Brillouin.
Experimenta podcast (in German) about light and its use in biology
10/05/2023In the podcast #10 for a general audience (German only), Robert talked about the nature of light and its use in biology.
Júlia Ferrer Ortas joining our lab as ARISE fellow
01/05/2023Júlia will be working on multi-photon microscopy and adaptive optics. Welcome Júlia!
Robert was interviewed by the IMP Vienna
12/04/2023Robert shared his thoughts and experience during his scientific career in an alumni portrait.
Our work on Line-Scanning Brillouin Microscopy is out
03/04/2023Our work on designing and using a line-scanning Brillouin microscope to image embryo development is online. You can read about it in the EMBL news, ORF.at, ANSA, le Scienze and Physics world. Congratulations to all people involved!
Samuel Davis joining our lab as ARISE fellow
01/04/2023Sam will be working on high-speed and multi-modal volume imaging of living organisms. Welcome Sam!
People from the Ströhl group (UiT University - Tromsø) visited us for 6 months
31/03/2023Florian Ströhl, Ida Opstadt, Jon-Richard Sommernes left after 6 months of visit at our lab to work on oblique plane microscopy, in collaboration with the Ikmi lab.
Our work on three-photon microscopy is featured in Nature news
07/02/2023Nature mentioned our work on three-photon microscopy for mouse brain imaging in a Technology Feature.
Our paper on oblique plane microscopy is out
10/01/2023Raj's and Kaushik's work on designing and using an oblique plane microscope for mesoscopic imaging of nematostella is online. Congratulations to all people involved!
Fan is leaving the lab
01/01/2023After more than 2 years, Fan is leaving us to take up a Faculty appointment at the Shanghai Institute of Optics and Fine Mechanics at the Chinese Academy of Sciences. Congratulations Fan and all the best for your new position!
Our Brillouin microscopy work was highlighted by the Guardian!
22/12/2022We're humbled and proud that recently our work on Brillouin microscopy was selected by the Guardian as as one of the 10 biggest science stories of 2022 (Check out #7). Congrats to the whole team!
Carlo defended his PhD thesis!
25/10/2022Congratulations to Carlo for an outstanding PhD defense!
Our OCM platform contributes to new paper by Ikmi group
16/09/2022Ling's Optical Coherence Microscope has been instrumental in a new interdisciplinary study that looks at the connection between animal behaviour and morphogenesis. Congratulations Anniek, Ling and everyone involved!
We are back from lab retreat
02/09/2022We have been in the Ardèche region in France. There we had the opportunity of having good scientific talks and discussions, visiting caves and enjoying the beautiful Ardèche river. Have a look at the group picture we took there!
Raj defended his PhD thesis!
15/05/2022Congratulations to Raj for an outstanding PhD defense!
Raj is leaving the lab
10/05/2022Raj is leaving the lab and joining LaVision. It was great to have you in the lab and all the best for your new job!
Our paper on optimising optical photoacoustic detection is out
05/04/2022Jakub's paper on Comparing free-space and fiber-coupled detectors for Fabry–Pérot-based all-optical photoacoustic tomography was published today! Congratulations to Jakub!
Gretel Kamm joining our lab as postdoc
01/04/2022Gretel will be working on investigating the neurobiology of sickness in mice. Welcome Gretel!
Two visitors joining our lab
10/02/2022Olek and Jing joined our lab to work on deep tissue imaging. Welcome Olek and Jing!
Our Brillouin microscopy work was featured by Labor Journal
07/02/2022The German scientific newspaper 'Labor Journal' published a nice and extensive article on Brillouin microscopy, featuring the work from our lab over the last years.
We are contributing to the 5th BioBrillouin meeting
11/10/2021We are contributing again to the annual BioBrillouin conference and training school series, with lectures by Robert, and training demos and a research presentation by Carlo.
We are at 'Seeing is believing'
04/10/2021The lab is well represented at the EMBL-EMBO Symposium 'Seeing is Believing'. Listen to Robert’s and Jakub’s talk as well as Carlo’s poster on Thu. Oct. 6.
Our paper on adaptive optics three-photon microscopy for deep brain imaging is out
30/09/2021Lina’s paper on adaptive optics three-photon microscopy for deep brain imaging was published today in Nature Methods! Congrats to the entire team - and special thanks to our wonderful collaborators!
We are joining the Interdisciplinary Center of Neurosciences
18/08/2021Our lab is joining the Interdisciplinary Center of Neurosciences (IZN) in Heidelberg with Robert as an Investigator. This will foster our local connections and we’re looking forward to future collaborations, some of which we’ve already started!
Today is the 5th anniversary of the lab!
01/08/2021Today marks the 5-year of our lab at EMBL. It’s been a wonderful journey so far and were are excited to see what the future has in store for us!
Lina is leaving the lab
01/07/2021Lina is leaving the lab. It was great to have you in the lab and all the best for your future!
Octave Martin joining our lab as PhD student
21/06/2021Octave will be working on using Deep Learning to combine Light Field and Light Sheet microscopy modalities for fast and accurate volumetric reconstruction with continuous validation. Welcome Octave!
We are part of a COST Innovator grant together with our collaborators of the ‘BioBrillouin’ network
28/05/2021We’re participating in a successful COST Innovator grant that aims at generating a comprehensive Brillouin spectral database of biological materials, together with our collaborators of the COST Action ‘BioBrillouin’ network.
New project funded by the MOLIT gGmbH
14/05/2021We received funding from the MOLIT gGmbH to develop 4D imaging methods for tumor organoids as part of our close collaboration with the Jechlinger lab.
Mehmet’s paper is now online
11/05/2021Mehmet’s paper on intravital cancer monitoring using fluorescence tomography, in collaboration with the Jechlinger lab, is now online at Communication Biology. Congratulations to everyone involved!
Our deep-learning light-field microscopy paper is out
07/05/2021Our deep-learning light-field microscopy paper is out in Nature Methods today! Congrats to the whole team and thanks to all our wonderful collaborators!
Ivo Leite joining our lab as postdoc
05/05/2021Ivo will be working on wavefront shaping and photoacoustics. Welcome Ivo!
Great news from the DFG!
28/04/2021Our lab will be funded for 3 years to work on revealing the neuronal population code of auditory space!
Jakub defended his PhD thesis!
25/03/2021Congratulations to Jakub for an outstanding PhD defense!
Amr Tamimi joining our lab as postdoc
08/02/2021Amr will be working on multiphoton microscopy. Welcome Amr!
Lina's presentation at the Optical Interest Group Seminar Series is online
03/02/2021Lina's presentation at the Optical Interest Group Seminar Series on 'Adaptive optics and three-photon microscopy' is now available online.
Lina defended her PhD thesis!
29/01/2021Congratulations to Lina for an impeccable thesis defense!
Marina Sabbadini joining our lab as PhD student
25/01/2021Marina will be working on mouse facial expression analysis in correlation with neuronal activity. Welcome Marina!
New master student joined our lab!
21/01/2021Ivana will be working on Brillouin microscopy. Welcome Ivana!
Congratulations to Joe for his new PI position!
20/12/2020After 5 years at EMBL Chii Jou (Joe) Chan is leaving to take up a group leader position at the Mechanobiology Institute in Singapore. It’s been great having him in the lab and collaborating on new applications of Brillouin microscopy. All the best for your new lab and scientific endeavours!
Robert's presentation at SPAOM is online
18/12/2020Robert's recent invited presentation at SPAOM 2020 on 'Light-field microscopy' is now available online.
Jakub's paper is out in Advanced Optical Materials
11/12/2020Jakub’s paper on “Improving sensitivity of Fabry-Perot based sensors using adaptive optics” is out in Advanced Optical Materials. Congratulations Jakub!
New master student joined our lab!
07/12/2020Emanuel will be doing a master thesis on image restoration. Welcome Emanuel!
New project funded by the Chan Zuckerberg Initiative
02/12/2020We are thrilled to receive funding from the Chan Zuckerberg Initiative to support a new project on deep-tissue imaging. We’ll work together with Claire Deo (EMBL Heidelberg) and Sylvain Gigan (Sorbonne Univ.) to advance fluorescence imaging deep inside scattering samples.
Nikita Kaydanov joining our lab as PhD student
21/10/2020Nikita started his PhD to work on photoacustic tomography. Welcome Nikita!
Fan Yang joining our lab as postdoc
07/09/2020Fan will be working on Brillouin microscopy. Welcome Fan!
Congratulations to Tristan!
07/08/2020Today Tristan successfully defended his MSc thesis. Good job Tristan!
Chii Jou Chan joining our lab as postdoc
01/06/2020Joe is joining our lab to work on studying mouse ovaries using Brillouin microscopy. Welcome Joe!
Kaushik joining as EIPOD postdoc between our and Aissam Ikmi’s lab
01/04/2020He will use some of our developed imaging technologies to understand morphogenesis and behaviour in Nematostella. Welcome Kaushik!
Excellent news from Brussels again!
25/03/2020We are receiving a Horizon 2020 FET Proactive grant to work on deep brain imaging using novel superconducting detector arrays developed by our project partners in Sweden and the Netherlands.
EMBL closure due to the Coronavirus pandemic
19/03/2020As of today, EMBL has closed their campuses across Europe, and thus our lab is now working from home. We’re going to miss our optics lab and mutual interactions, but hope everyone stays safe and healthy - see you soon!
Raj, from the Hufnagel lab, is joining our lab
01/03/2020Raj will be working on new light-sheet imaging approaches. Welcome Raj!
Robert is back from an exciting week in the Bay Area
07/02/2020A Neurotechnology Plenary at SPIE Photonics West, as well as invited presentations and visits to UC Davis and the CZI Biohub.
New visiting student joined our lab!
07/01/2020Sebastian will be working on real time images process and hardware control. Welcome Sebastian!
We are now part of the Molecular Medicine Partnership Unit
19/12/2019We are now part of the Molecular Medicine Partnership Unit (MMPU) between the University of Heidelberg and EMBL. Together with our colleagues Rohini Kuner and Jan Siemens from the University and Theodore Alexandrov from EMBL we are part of the group “Chronic Pain & Homeostasis” and will contribute with our deep in-vivo brain imaging expertise.
Great news from Brussels!
10/12/2019The lab has been awarded with an ERC Consolidator grant to fund our work on Brillouin microscopy for the next 5 years. We are all excited and will be looking for new lab members soon.
Lina is at the International Workshop on Adaptive Optics in Industry and Medicine
21/10/2019On the 24th of October, she will present a talk about her recent progresses in adaptive optics and multi-photon microscopy.
Official websiteNew Master student joined our lab!
14/10/2019Tristan, from University of Heidelberg, will be doing his master thesis on analysing facial expressions in mice in relation to motor cortex activity. Welcome Tristan!
Mesoscopic Fluorescence Tomography paper appeared in Biomedical Optics Express
11/10/2019The paper investigates different Source Detector configurations for mesoscopic fluorescence molecular tomography. Check it out!
Link to the paperWe are at "Seeing is believing"conference at EMBL!
09/10/2019Carlo, Jakub and Lina are presenting a poster on the 9th and on the 11th of October.
Official websiteRobert at the Interdisciplinary Symposium on 3D Microscopy
01/10/2019Robert is presenting this week at the Interdisciplinary Symposium on 3D Microscopy in Engelberg, Switzerland, organized by the Swiss Society for Optics and Microscopy
Official websiteWe are in Porto for the 3rd BioBrillouin meeting
17/09/2019On Thursday 26th September Carlo is presenting our work on "Imaging mechanical properties of sub-micron ECM in live zebrafish using Brillouin microscopy"
Here you can find more infoWe are back from lab retreat
27/08/2019We have been, togheter with the Diz-Muñoz group, close to the Neckar river. It was a retreat with good scientific talks and discussions and exciting social activities like canoing and geo-cashing. Have a look at the picture before canoing!
Congratulations to Momoko!
10/07/2019Today Momoko successfully defended her MSc thesis on "Development of a serial time-encoded interferometer for photoacoustic imaging". Well done Momoko!
DFG grant approved
1/07/2019Happy to announce that we got DFG funding to join SPP 1926 on voltage imaging. Looking forward to working with Paul Heppenstall and the entire consortium
We are at ECBO in Munich
22/06/2019On Tuesday 25 June Mehmet is presenting "Longitudinal monitoring of in-vivo mice mammary tumor progression using intravital fluorescence tomography and optical coherence tomography"
Here you can find more infoIso-LFM paper is published in Nature Methods
29/04/2019Our work on dual-view light-field imaging of fast biological processes appeared online today in Nature Methods. Congrats to everyone involved!
EMBL also published an article and video describing our work. Check it out!
New postdoc to join our lab on neuroimaging
25/03/2019Juan Boffi joins us as an EIPOD postdoc from the Kuner lab in Heidelberg. Juan will use our fast volumetric microscopes to study sensory processing in the mouse brain. Welcome Juan!
Jakub is at EMIM in Glasgow
19/03/2019Tomorrow at 16:00 he is presenting a poster "Longitudinal studies of mouse mammary gland tumour models using photoacoustics"
Here you can find more infoWe are online!
08/03/2019Today our website is online!














European Molecular Biology Lab
Cell Biology and Biophysics Unit
Developmental Biology Unit
Epigenetics and Neurobiology Unit
Meyerhofstraße 1
69117 Heidelberg
Germany
Room 407
+49 6221 387 8722
prevedel(at)embl.de